Thursday, May 20, 2021
Ubigene Biosciences Gene Point Mutation/ Knockin Cell Line service , Comes with 2000 USD Cell Assay Coupon
 Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Gene Knockout cell line, 100% WB guarantee Only 3780 USD | Ubigene Biosciences
 Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Friday, May 14, 2021
Application of KO cells | Ubigene Biosciences
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), the term refers to a series of repetitive patterns in the DNA of bacteria and archaea that were extensively studied by Spanish scientist Francis Mojica in the ‘90s. These patterns are the basis of a primitive immune system that bacteria use to ‘remember’ the DNA of viral invaders by incorporating the DNA sequence of the virus within the CRISPR patterns. The Cas9 protein is then able to recognize the DNA sequence stored within CRISPR patterns and cut any DNA molecules with a matching sequence. Since 2012, the CRISPR/Cas systems was developed into easy-to-use and robust genome editing tool for basic research and clinics.
Three common strategies have been developed for genome editing with the CRISPR/Cas9 platform:
(1)The plasmid‐based CRISPR/Cas9 strategy, where a plasmid is used to encode Cas9 protein and sgRNA, assembles Cas9 gene as well as sgRNA into the same plasmid in vitro. However, the encoded plasmid needs to be introduced inside the nucleus of target cells, which is a key challenge in this system.
(2)Direct intracellular delivery of Cas9 messenger RNA (mRNA) and sgRNA, the greatest drawback of which lies in the poor stability of mRNA, which results in transient expression of mRNA and a short duration of gene modification;
(3)Directly delivery of Cas9 protein and sgRNA, which has several advantages, including rapid action, great stability, and limited antigenicity. Among the versatile CRISPR-based tools, CRISPR/Cas9 is the most widerly used one for studying gene functions. It has boost the generation of knockout cell lines for different applications.
Knockout cellular model applications:
There is a significant challenge in translating the abundance of genetic information. To date, it is possible to identify the role of genes, to understand basic biology, as well as linking to the role of mutations for understanding disease pathogenesis. A Knockout (KO) cell lines are excellent model systems to do this. The advantage of cell lines is the ability to use gene-editing to construct isogenic cell line pairs, where a mutant model can be probed alongside a wild-type control. The following are potential examples of how to get the most out of your research using KO cell lines:
1. Antibody specificity validation
2. Identify pathway players
3. Identify drug targets
4. Build disease models
5. Develop therapeutic tools
1. Antibody specificity validation
The research community believes that the use of excellent research tools impacts significantly the quality of research delivered. Antibodies are one of the most commonly used reagents in life-sciences, but despite their widespread use, there are no standard guidelines for how these invaluable biological tools should be validated prior to use. For example, poorly characterized antibodies may yield non-specific results, which are difficult to replicate even when the only difference is the antibody's production lot. However, the bar for antibody validation is rising and there are calls for the industry standard to show application-specific validation using KO cell lines. So, KO cell line has wide application in antibody validation. The parallel use of wildtype and KO cell lines provides a valuable tool to control for research reagents quality.
2. Identify pathway players
Often there are multiple related, but distinct molecules and processes present in a pathway. Gene-edited cell lines are a well-established tool to probe the role of a particular gene or mutation. For example, JAK1 is essential for signaling for certain cytokines and is thought to play a role in tumor metastasis. It is also known to have a functional and physical association with INF gamma receptors. When ligands bind with receptors, the receptor-associated JAKs become activated, leading to the recruitment of specific STATs (signal transducer and activator of transcription) from the cytoplasm. These STATs then become JAK substrates. Activated STATs are released from the receptor, which then translocates to the nucleus to bind to specific enhancer elements. These then affect the expression of target genes. The role of JAK1 in STAT phosphorylation can be confirmed by creating a frameshift mutation in the JAK1 gene and the KO cell line do not allow phosphorylation of STAT and this could be verified with westernblot analysis.
3. Identify drug targets
Pharmacological interventions are commonly used to interrogate pathways. For example, the chemotherapeutic 6-thioguanine (6-TG) is used to probe the effect of DNA damage response. To maintain genomic integrity, cells are equipped with a myriad of mechanisms that are each specific for different types of damage. The efficiency of these DDR pathways plays an essential role in the effectiveness of cytotoxic treatments. 6-TG is a growth inhibitory antimetabolite that requires an active DNA mismatch repair (MMR) system to be effective. One study showed that HPRT+ cells are sensitive to 6-thioguanine (6-TG), which can be converted to the nucleotide form by HPRT and incorporated into DNA by DNA polymerase, killing cells by a process involving postreplicative mismatch repair. The strategy is to transfect cells with two plasmids that express respectively a HPRT guide RNA and a guide RNA for the gene of interest. Cas9 can be expressed from the gene on a separate plasmid, a plasmid carrying the HPRT gRNA or integrated into the chromosome (if such a cell line is already available). If a cell becomes resistant to 6-TG, it would suggest that this cell should also be competent to target the gene of interest as long as the gRNA is effective. Thus if the targeted gene is not altered in the resulting 6-TG resistant cells, it would suggest that the guide RNA is ineffective. On the other hand, if no 6-TG resistant cells can be obtained by co-targeting, it would suggest that the gene of interest might be essential. Thus KO cell lines that are devoid of key proteins are used to confirm the target of the 6-TG.
4. Build disease models
After identifying mutations of interest in a patient population, the next steps are to probe the biological mechanisms that result in the disease phenotype or the response to treatment. KO cell lines, combined with simple molecular biology techniques, allows the researcher to rescue the gene of interest to restore phenotype and complement the Knockout cell line with a functional mutation, such as a point mutation observed in a patient population. One example of disease modeling is MAGEC2 knockout in melanoma cancer cell line. MAGEC2, a member of the type I melanoma-associated antigen family, is expressed in a wide variety of cancer types but not in normal somatic cells. Reserchers generated MAGEC2-knockout A375 melanoma cell lines using the CRISPR/Cas9 system. They found that knockout or knockdown of the MAGEC2 gene sensitized melanoma cells to tumor necrosis factor-α-induced apoptosis. Their study sheds a light on the molecular pathway by which MAGEC2 promotes tumor development.
Earlier studies reported that Nuclear Factor Erythroid 2-Related Factor (NRF2) is a master regulator of 100–200 target genes involved in cellular responses to oxidative and/or electrophilic stress. NRF2 is also known to regulate the expression of genes involved in protein degradation and detoxification, and it is negatively regulated by Kelch-like ECH-associated protein 1 (KEAP1), a substrate adaptor for the Cul3-dependent E3 ubiquitin ligase complex. One research group used CRISPR/Cas9 to disable the NRF2 gene in lung cancer cells by disrupting the NRF2 nuclear export signal (NES) domain; phenotypically, the protein is largely blocked from transiting into the nucleus after translation. Cells with this gene knockout were found to have a reduced proliferation phenotype and are more sensitive to chemotherapeutic agents, such as cisplatin and carboplatin. They also found that homozygous knockout cells proliferate at a slower rate than the wild-type cells, even in the absence of drug treatment.
5. Develop therapeutic tools
During the past few years, the team represented by China and the United States have conducted a series of clinical trials of gene editing, such as producing more effective CAR T cells for the treatment of cancer and the knockout of the erythroid-specific enhancer of BCL11A to upregulate gamma globulin in autologous erythroid HSCs as a potential therapy for sickle cell disease and β-thalassemia. To date, several ongoing clinical trials applying the concept of PD-1 knockout autologous T cells to treat cancers, including prostate cancer (NCT02867345), esophageal cancer (NCT03081715), and renal cell cancer (NCT02867332). These trials are considered proof-of-concept studies to apply the in vitro CRISPR/Cas9 gene knockout technique in cancer therapy.
In summary, KO cell lines are a versatile tool for helping us understand basic biology, as well as identifying the role of mutations in disease pathogenesis. Ultimately, this has led to advances in disease treatment and patient prognosis.
Reference:
Enriching CRISPR-Cas9 targeted cells by co-targeting the HPRT gene. Nucleic Acids Res. 2015. 43(20): e134.
Establishment of MAGEC2-knockout cells and functional investigation of MAGEC2 in tumor cells. Cancer Sci 2016. 107(12):1888-1897.
Functional Gene Knockout of NRF2 Increases Chemosensitivity of Human Lung Cancer A549 Cells In Vitro and in a Xenograft Mouse Model. Molecular Therapy: Oncolytics. 2018 11:75-89.
Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther .2020 Jan 3;5(1):1.
 Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Monday, May 10, 2021
[One-Stop Services] Cell Assay Services | Ubigene
A subsequent series of cell assays are important as well! But!
No equipment to do the cell-based assays for phenotypic analysis?
Too messy and huge workload for data analysis?
No appropriate service provider found?
Ubigene have now launched a series of new services - Cell assays
Providing One-Stop Services from cell line generation to cell phenotypic analysis
Ubigene can help save your time from tedious, repetitive experiments and obtain
the experimental data for you.
Here is the big promotion for you!
As long as you have ordered any KO / Overexpression / Knockdown Cell Line project from Ubigene, you can enjoy the promotional price for the following all-in-one package of cell assays.
Promotional package: cell subculturing, cell proliferation assay, cell migration or invasion assay, and cell cycle assay.
 Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Thursday, May 6, 2021
Cell migration and invasion-Principles and Methods | Ubigene
Cell migration and invasion
Cell migration, also known as cell crawling, or cell movement, refers to the movement of a cell after receiving a migratory signal or sensing a gradient of certain substances. Cell migration is an alternating process of pseudopodia extension at the cell head, the establishment of new adhesions, and retraction of the cell body tail in a spatiotemporal manner. Cell migration, one of the basic functions of normal cells, is a physiological process of normal growth and development of the organism, and a ubiquitous form of movement of living cells. Cell migration is implicated in processes such as embryonic development, angiogenesis, wound healing, immune responses, inflammatory responses, atherosclerosis, and cancer metastasis.
Cell invasion refers to the ability of cells to migrate from one area to another through the extracellular matrix. Cell invasion is a response of normal and cancer cells to chemical and mechanical stimuli. Before migrating to new areas, the extracellular matrix is degraded by proteases within the cell. Cell invasion often occurs during wound repair, vascularization and inflammatory response as well as abnormal tissue infiltration, tumor cell metastasis, and so on.
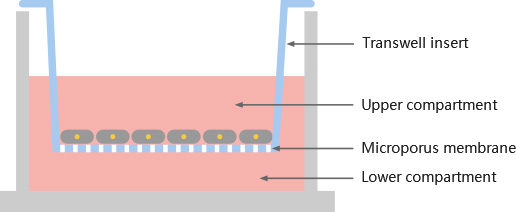
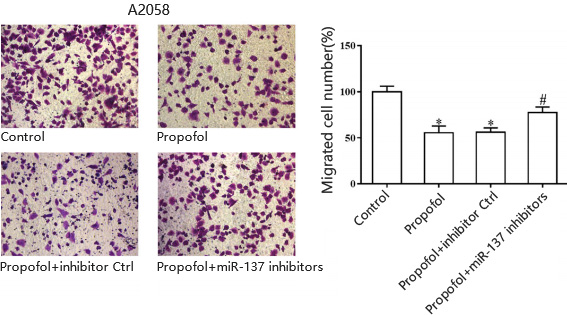
A.Cell migration for human skin melanoma cell line A20578
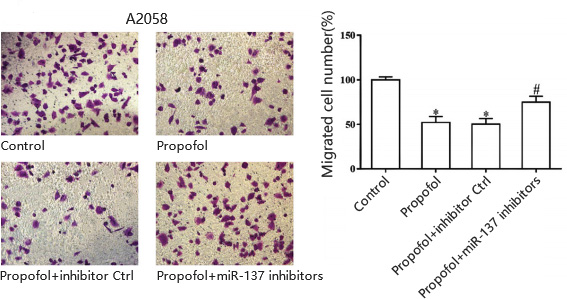
B.Cell invasion for human skin melanoma cell line A20578
Reference
Yu, Hong et al. “Propofol suppresses proliferation, invasion, and migration of human melanoma cells via regulating microRNA-137 and fibroblast growth factor 9.” Journal of cellular physiology vol. 234,12 (2019): 23279-23288. doi:10.1002/jcp.28896
Service turnaround
5~10 business days, depending on cell growth and experimental design
Customer provides
cell lines, drugs, drug treatments and experimental conditions parameter settings;
Deliverables
cell lines, drugs, drug treatments and experimental conditions parameter settings;
 Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Wednesday, May 5, 2021
Cell Proliferation-Principles and Methods | Ubigene


Reference
Deng, Danni et al. “p62 acts as an oncogene and is targeted by miR-124-3p in glioma.” Cancer cell international vol. 19 280. 6 Nov. 2019, doi:10.1186/s12935-019-1004-x
Service turnaround
5~10 business days, depending on cell growth and experimental design
Customer provides
cell lines, and experimental conditions parameter settings
Deliverables
raw data, analytical results, experimental reports
 Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
Ubigene Biosciences, is a fast-growing company with an experienced team in gene-editing. Our advanced molecular and cell biology platform ensure us to provide high-quality services and products for biological research and biotechnology industries.
[Research highlight] Enhancing p53 pathway can efficiently suppress colon cancer
Colorectal cancer is the third most diagnosed cancer and leads to the second mortality among cancers worldwide. The first-line chemotherap...
-
Colorectal cancer is the third most diagnosed cancer and leads to the second mortality among cancers worldwide. The first-line chemotherap...
-
Background In 1996, U-87 MG cell line was isolat ed from the malignant glioma of a 44-year-old female patient. This human primary gliob...







