Escherichia coli (also known as Escherichia coli) is a Gram-negative, facultative anaerobic, rod-shaped coliform of Escherichia coli, usually found in the lower intestine (endothermic) of warm-blooded organisms. Most E. coli strains are harmless, but certain serotypes can cause severe food poisoning in the host, and sometimes product recalls due to food contamination. Harmless bacterial strains are part of the normal intestinal flora and can have a symbiotic relationship by producing vitamin K2 and preventing pathogenic bacteria from colonizing in the intestine, thereby benefiting the host. Escherichia coli is excreted in feces. The bacteria grew in large numbers in fresh feces for 3 days under aerobic conditions, but then the number slowly decreased. Due to the long history of laboratory culture and easy operation, Knockout Cell Lines E. coli plays an important role in modern bioengineering and industrial microbiology. [82] The work of Stanley Norman Cohen and Herbert Boyer in E. coli, using plasmids and restriction enzymes to produce recombinant DNA, became the basis of biotechnology.
Metabolic flux analysis of pykF knockout Escherichia coli based on 13C labeling experiment and measurement of enzyme activity and intracellular metabolite concentration
Metabolic flux analysis based on 13C labeling experiment was carried out, and then 2D NMR and GC-MS were used to measure intracellular isotope distribution to study the effect of pyruvate kinase (pyk) gene knockout on the metabolism of continuously cultured E. coli. In addition, in batch culture and continuous culture, the activity of 16 enzymes and the concentration of 5 intracellular metabolites were measured as a function of time. It was found that the flux through phosphoenolpyruvate carboxylase and malate was up-regulated in the pykF-mutant compared to the wild-type, and the formation of acetate was significantly reduced in the mutant. In addition, in the mutant, the flux through the phosphofructokinase pathway was reduced, while the flux through the oxidized pentose phosphate (PP) pathway was increased. This is evidenced by the corresponding enzymatic activity and increased concentrations of phosphoenolpyruvate, 6-phosphate glucose and 6-phosphate gluconate. It was also found that for continuous culture, the enzyme activity of oxidizing PP and Entner-Doudoroff. The dilution of the pykF-mutant increases with the increase of the pathway. In order to clarify the metabolism quantitatively, it is important to find that it is important to integrate information about the intracellular metabolic flux distribution, enzyme activity and intracellular metabolite concentration.
Using CRISPR-Cas9 to reconstruct the TCA cycle involving I-isoleucine dioxygenase to hydroxylate I-isoleucine in E. coli
L-isoleucine dioxygenase (IDO) is an iron (II)/α-ketoglutarate (α-KG) dependent dioxygenase, which can convert l-isoleucine (l-Ile) Specifically converted to (2S, 3R, 4S)-4-hydroxyisoleucine (4-HIL). 4-HIL is an important drug for the treatment and prevention of type 1 and type 2 diabetes, but the current method has a low yield. In this study, the CRISPR-Cas9 gene editing system was used to knock out the sucAB and aceAK genes in the TCA cycle pathway of E. coli. For single gene knockout, the entire process takes about 7 days. However, the time for each round of genetic modification for multiple rounds of editing was reduced by 2 days. Using the genome-edited recombinant strain Escherichia coli BL21(DE3)ΔsucABΔaceAK/ pET-28a(+)-ido(2Δ-ido), compared with E., the biotransformation rate of 4-HIL by L-Ile increased by about 15% . Escherichia coli BL21(DE3)/pET-28a(+)-ido [BL21(DE3)-ido] strain. The CRISPR-Cas9 editing strategy has the potential to modify multiple genes more quickly and optimize strains for industrial production.
CRISPR-Cas9 knocking out qseB induces asynchrony between E. coli motility and biofilm formation
Generally, cell motility and biofilm formation are tightly regulated. The QseBC two-component system (TCS) acts as a bridge for bacterial signal transmission, where the QseB protein acts as a response regulator of bacterial motility, biofilm formation and virulence. In general, the mechanism that controls the interaction between QseBC and its functions has been studied, but it is not clear how QseB regulates bacterial motility and biofilm formation. In this study, the E. coli MG1655ΔqseB strain (strain ΔqseB) was constructed using the CRISPR-Cas9 system, and the influence of the qseB gene on wild-type (WT) motility and biofilm formation was determined. The results of vigor determination showed that the ΔqseB strain had higher (p<0.05) vigor than the WT strain. However, there was no difference in biofilm formation between ΔqseB and WT strains. real-
Ubigene Biosciences is co-founded by biological academics and elites from China, the United States, and France. We are located in Guangzhou Science City, which serves as a global center for high technology and innovation. Ubigene Biosciences has 1000㎡ office areas and laboratories, involving genome editing, cell biology technology, and zebrafish research. We provide products and services for plasmids, viruses, cells, and zebrafish. We aim to provide customers with better gene-editing tools for cell or animal research.
We developed CRISPR-U™ and CRISPR-B™(based on CRISPR/Cas9 technology) which is more efficient than general CRISPR/Cas9 in double-strand breaking, CRISPR-U™ and CRISPR-B™ can greatly improve the efficiency of homologous recombination, easily achieve knockout (KO), point mutation (PM) and knockin (KI) in vitro and in vivo.
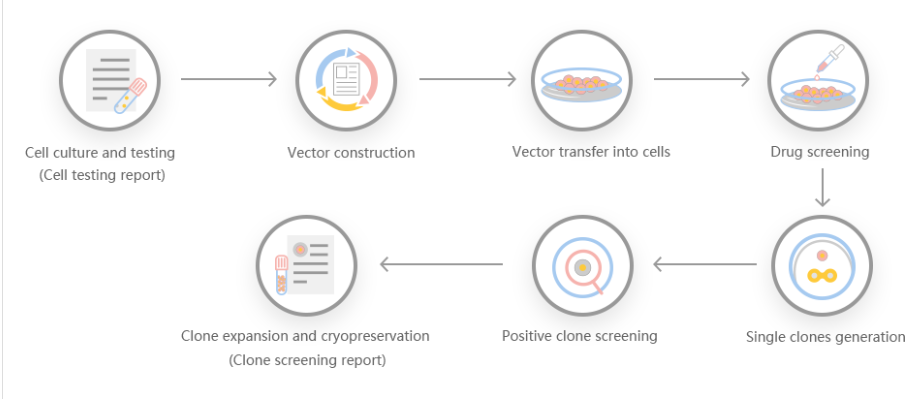
2. Provides different virus packaging services, including lentiviruses, adenoviruses and adeno-associated viruses.3. Provides high-quality services for gene knockout, point mutation and knockin cell lines.
2. Provides culture strategies and related products for different cell types.3. Provides cell biology-related services such as cell isolation, extraction and validation.

No comments:
Post a Comment